usp class vi materials
Excelon RNT 60 PVC Vinyl Lab Tubing. Newman USP Class VI O-Rings combine.

Usp Class Plastics Pacific Biolabs
Primarily biopharmaceutical manufacturers use USP Class VI for their process equipment.

. Yet some suppliers that. Pharmacopoeia XXII 1190 Class VI. A new line of inflatable seals which meet US Pharmacopoeia USP Class VI certification is now available.
USP Class VI materials meet the most stringent requirements and include silicones that pass a systemic toxicity test an intracutaneous test and an implantation test. As one of the most widely used methods VI forms part of six different classes with this being the most. Moldable polyurethanes Resilon 4300 and 4301 Molythane 4615 Machinable polymer-filled 0618 PTFE Life Sciences Capabilites.
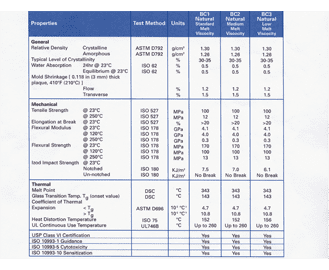
27 rows The USP Class VI compounds must be made from ingredients with clear histories of biocompatibility that meet tighter requirements for leachates. Our time-tested engineered proprietary materials that are certified the world over to meet the highest industry standards. Biocompatibility Information for Materials.
A number of our plastic materials are ISO-10993 or USP Class VI capable. USP stands for US. 1965 USP XVII introduced Biological TestsPlastics Containers section was added and made official in the Compendium.
The United States Pharmacopoeia USP 30 NF 25 2007 standard also known as Class VI is widely used to comply with stringent FDA regulations for products that come in. Typical applications for our FDA. The CVI Series meets the specific needs of pharmaceutical equipment.
In particular regarding the USP class VI certification process materials have to pass the biological tests ie. Excelon RNT 1065 Vinyl Tubing. Excelon RNT 68 Food Beverage Tubing.
USP Class VI Tests. USP Class VI Testing Methods. Plastics were assigned Class I-VI based on the biological in vivo.
Building trust in mRNA-based therapies. USP Class VI Approved Plastic Materials. It generally ensures a high quality level and better acceptance with the FDA and USDA.
In vivo testing USP. Pharmacopeia a private non-government organization that promotes the public health by establishing state-of-the-art standards to ensure the quality of medicines and. Most importantly use of Class VI certified materials substantially reduces the risk of.
SIMONA PP-H USP Class VI sheet is ideal for applications requiring biocompatibility testing standards defined by ISO 109931. For this reason the FDA provides a standard 21 CFR1772600 defining allowable rubber compound ingredients and extractibles based on toxicity and carcinogenicity. Our USP Class VI certified material offering includes.
Tests are based on. Class testing is frequently conducted on plastic materials that come in contact with injectable drugs and other fluids found in various steps of the drug manufacturing process. Fixes needed to prepare medicines supply chain for next crisis.
A selection of Figure 4 VisiJet Accura and DuraForm plastic materials. Something that is listed as being USP Class VI demonstrates that the materials utilized are biologically compatible when tested according to the US. SIMONA PP-H USP Class VI sheet material is easy to clean.
Class VI Gasket Material Options. C-Flex ULTRA biopharma pump tubing. Sterile and diaphragm valves have USP Class VI PTFE material in them and.
USP Class VI testing is conducted by producing an extract of the product. -55F -48C to 275F 135C short term to. In order to identify the biocompatibility of materials USP Class VI testing is required.
AdvantaFlex TPE Biopharma Tubing.

O Rings Fda And Usp Class Vi Darcoid Rubber Company Oakland California

Usp Class Vi And Biocompatibility Of Products For Pharmaceutical Use Mtg
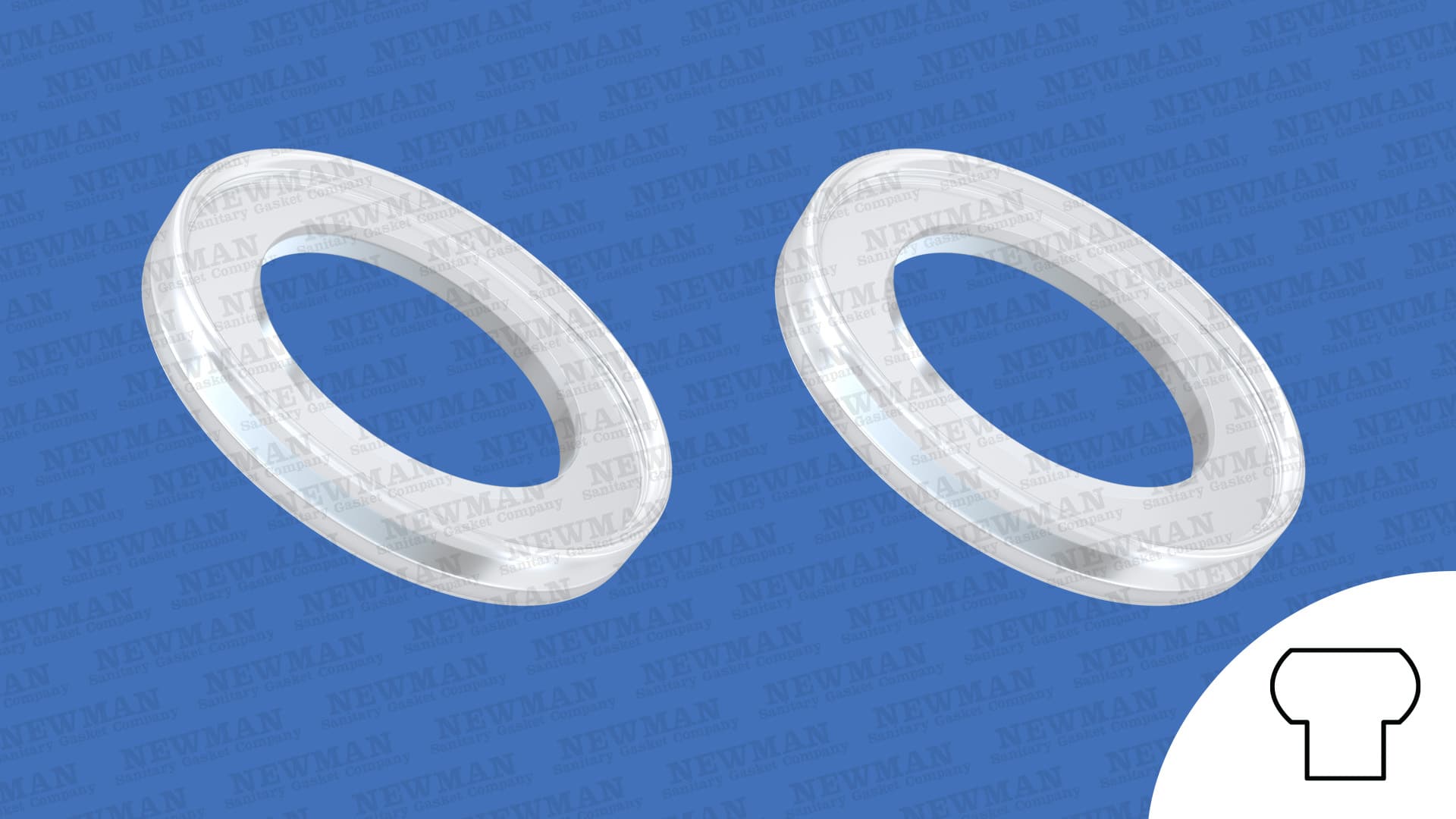
Biopharmaceutical Usp Class Vi Gaskets Newman Sanitary Gasket
Meaning Of Usp Class Vi Standard United Kingdom
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Material Selection Medical Injection Molding Xcentric Mold

Meaning Of Usp Class Vi Standard United Kingdom

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell
Dursan Passes Usp Class Vi Testing Why Is That Important
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Usp Class Vi Foster Corporation

Rulon 641 Usp Approved And Fda Compliant Tristar Plastics

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing





